Principle
This involves interfacial polymerization. Here, the two monomers are highly reactive at ambient temperatures and can form two immiscible liquid phases; one is aqueous and the other is organic. Then a condensation polymer is formed at the interface between the two phases. Interfacial polymerization can be of two types: Unstirred and stirred. In the unstirred process, the polymer formed at the interface can be pulled out in the form of a rope. But in the stirred process, small microcapsules or microspheres are produced.Reaction
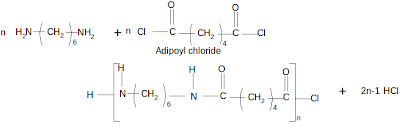
Nylon 66 is formed by the reaction between hexamethylene diamine and adipic acid. In order to get good yield, the reaction should be carried out at high temperature. Also the byproduct (water) should be removed continuously.Experiment
Adipoyl chloride is dissolved in an organic solvent (CCl4) taken in a beaker. The hexamethylene diamine is dissolved in water (containing a small amount of Na2CO3) in another beaker. This is carefully poured down the sides of the beaker containing the acid chloride. Care should be taken so that the amine solution does not fall straight into the acid chloride solution. The aqueous layer remains as the top layer over the denser organic layer. These layers are allowed to stand undisturbed. Then it can be seen that an opaque film is formed at the interface. The film formed can be pulled out of the beaker gently with a forceps as a continuous filament. This will continue till one of the reactants is consumed completely.
This
experiment is analogous to the famous Rope
Trick magic and so it is named as
Nylon Rope Trick.
Characteristics
Interfacial Polycondensation differs from bulk or solution polymerization in the following aspects.
- Reaction is fast at room temperature.
- No costly apparatus is necessary other than beakers, forceps etc.
- Diffusion of monomers into the interface determines the rate of reaction. This again depends up on the rate of the interface (film) removal.
- Interfacial Polycondensation possesses some of the properties of chain polymerization. i.e., the monomer reacts with the growing chains at the interface rather than it diffuses through the polymer film to initiate new chains. Hence the molecular weight is significantly high.
- The polymer product is formed at the interface while the byproduct (HCl or H2O) is dissolved in one of the phases. Thus it is removed from the reaction site.
- Because of the above reasons, equimolarity of bifunctional monomers is not necessary in interfacial polycondensation, which is an essential requirement in normal polycondensation reactions.